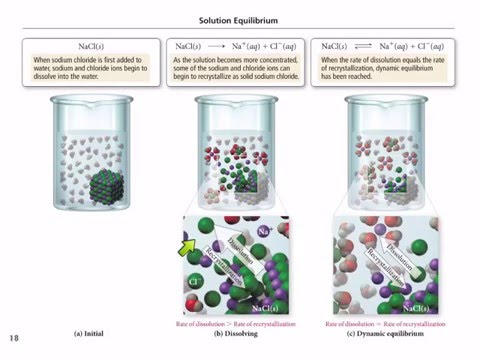
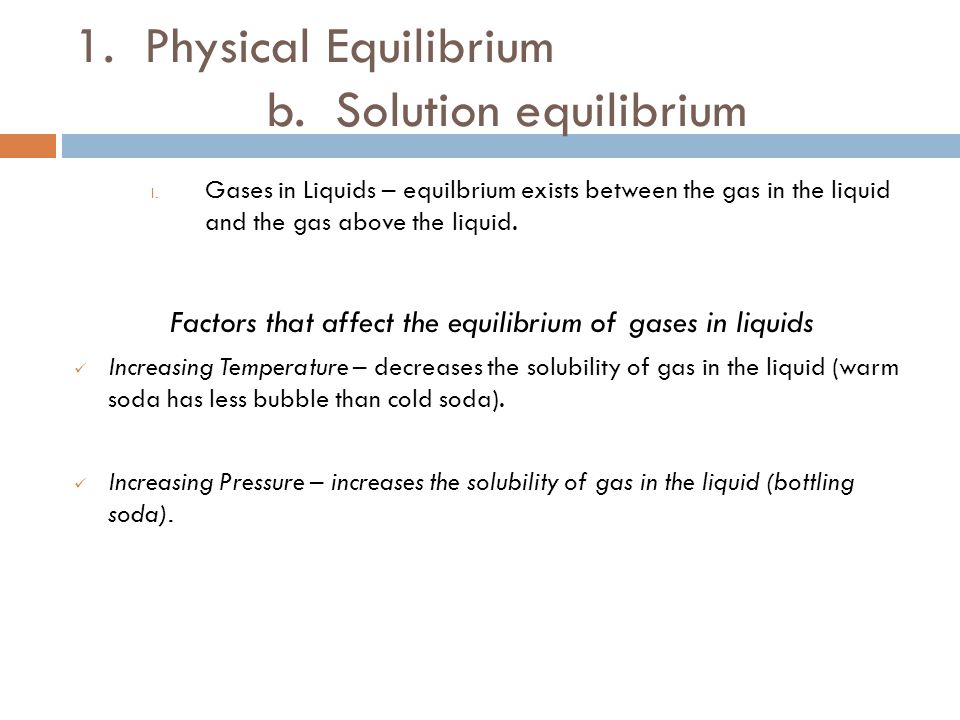
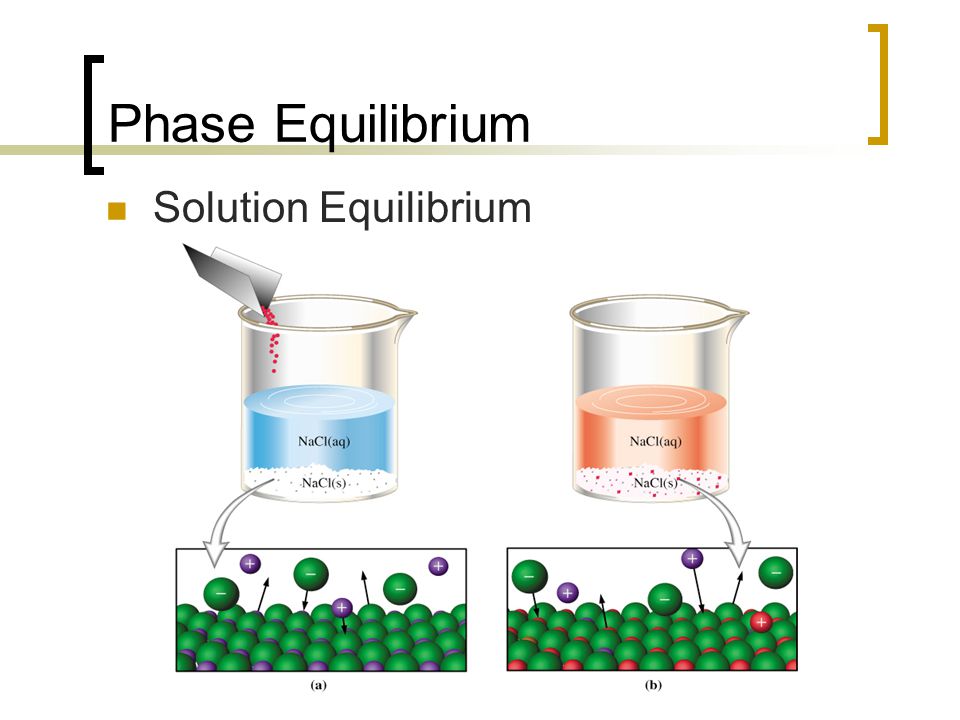
Solution Equilibrium Is Reached In The System When
Solution equilibrium is reached in the system when. A useful table of pK a values in DMSO solution has been compiled from the work of FG. Therefore equilibrium can be reached from either direction. Reached where the amounts of reactants and products no longer change.
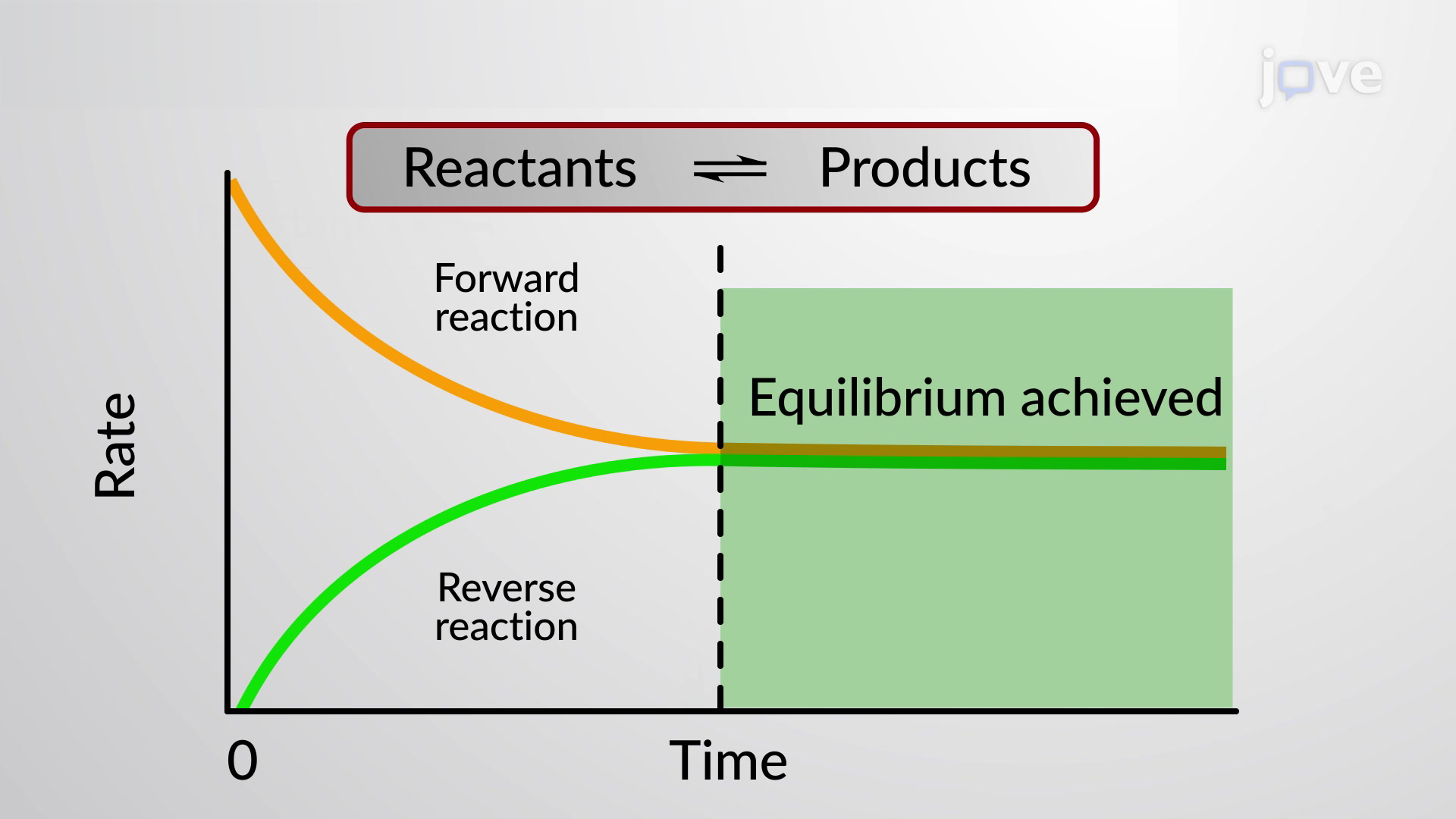
This state results when the forward reaction proceeds at the same rate as the reverse reactionThe reaction rates of the forward and backward. Ekwĭ-libre-um 1. If only ammonia is placed in the tank again a mixture of the three will occur.
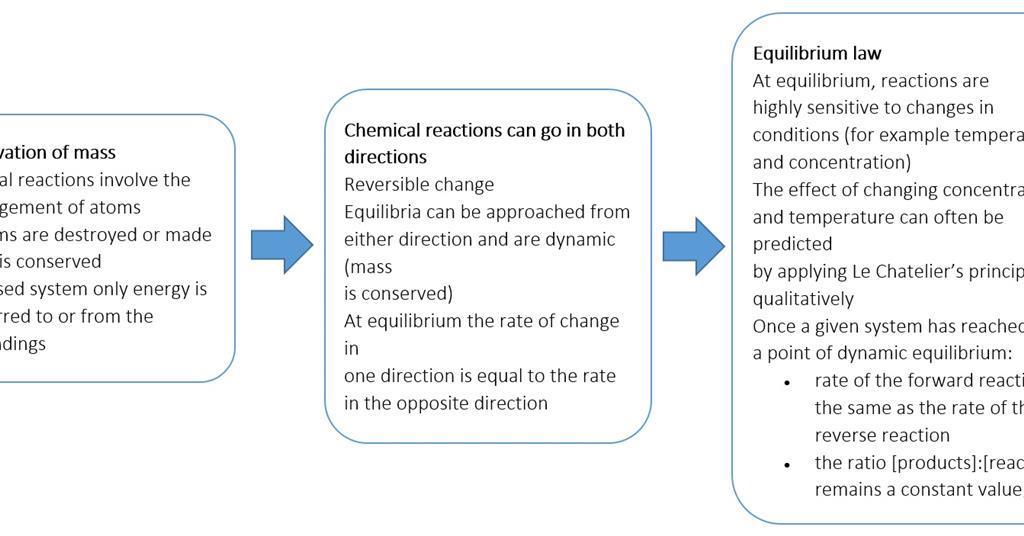
See also acid-base balance and fluid balance. Under such conditions the reactants and products are in chemical equilibrium and will remain so until the system is altered in some way. Reaching Equilibrium and the Equilibrium Position.
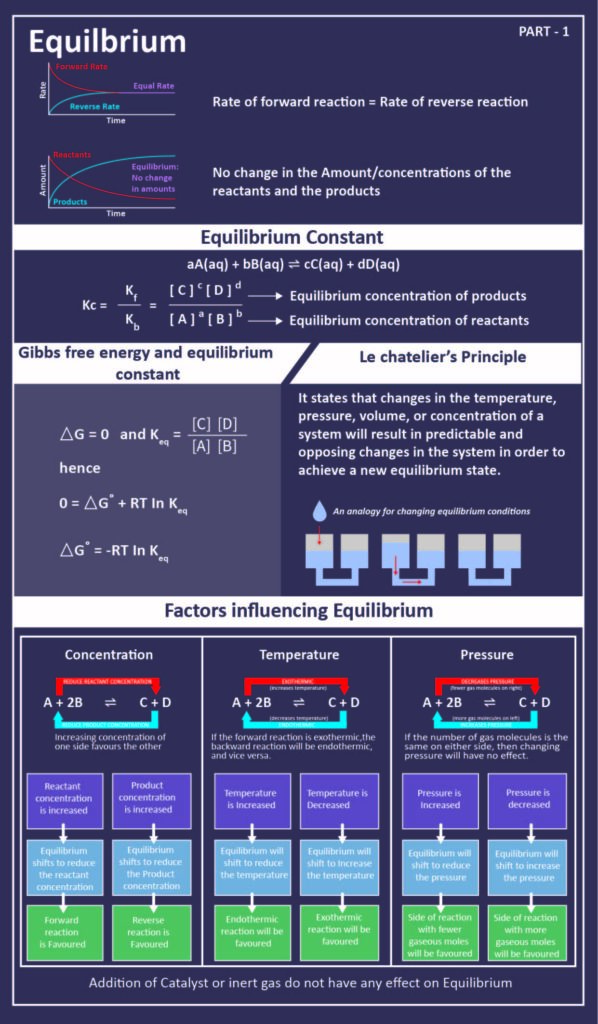
In the first call to the function we only define the argument a which is a mandatory positional argumentIn the second call we define a and n in the order they are defined in the functionFinally in the third call we define a as a positional argument and n as a keyword argument. Equilibrium Class 11 Notes Chemistry Chapter 7 Chemical Equilibrium In a chemical reaction chemical equilibrium is defined as the state at which there is no further change in concentration of reactants and products. System 2 has K ll 10-3 so the reactants have little tendency to form products under the conditions specified.
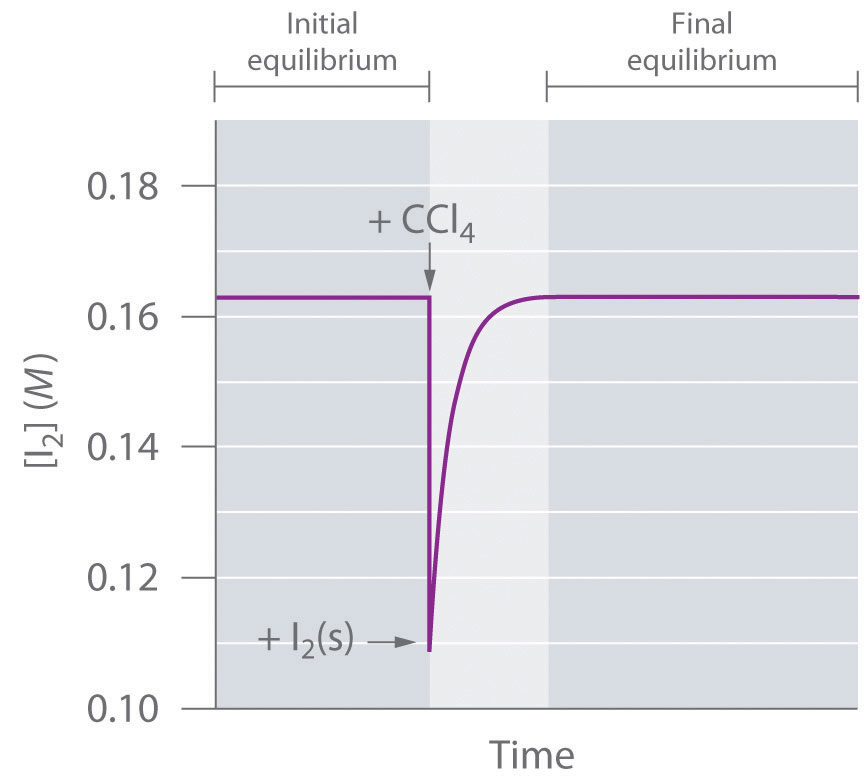
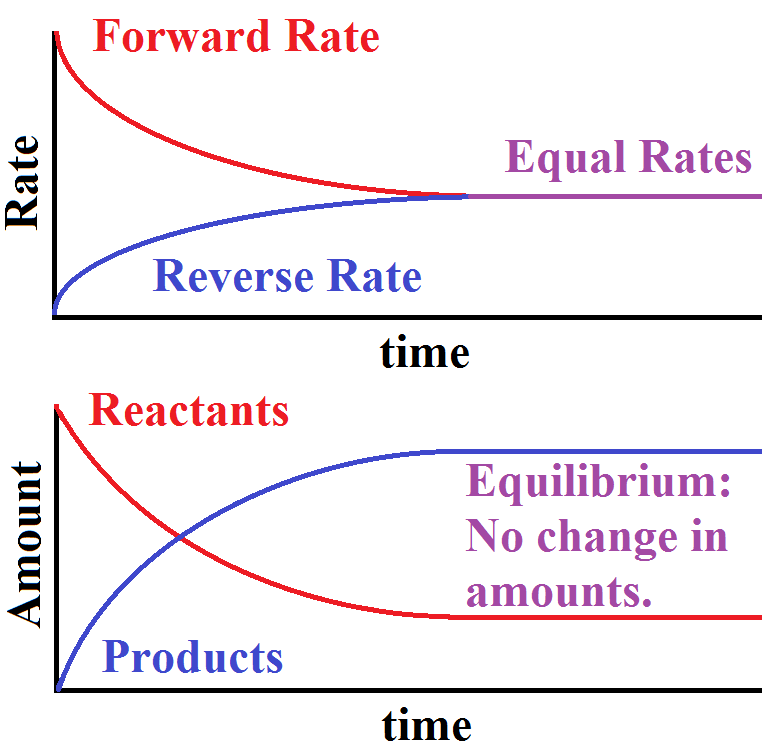
When the curve levels out and the concentrations become constant that time equilibrium state has been reached. The Equilibrium Constant A relationship between the concentrations of reactants and products at equilibrium can be determined. Other constants for dynamic equilibrium involving phase changes include partition coefficient and solubility product.
Harmonious adjustment of different elements or parts. A Nash Equilibrium is a set of strategies that players act out with the property that no player benefits from changing their strategy. After equilibrium was reached the concentration of PCl 5 was found to be 05 x 10-1 mol L-1.
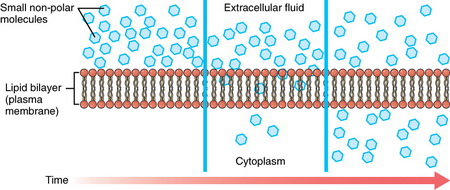
The ions will continue to flow into the cell until equilibrium is reached. By the time point U is reached all of the crystals of A have been remade to crystals of D.
This equilibrium is only established if the calcium carbonate is heated in a closed system preventing the carbon dioxide from escaping.
According to the Lewis theory an acid is an electron pair acceptor and a base is an electron pair donor. Reached where the amounts of reactants and products no longer change. Sense of equilibrium. Figure 9 shows. Reaching Equilibrium and the Equilibrium Position. The equilibrium produced on heating calcium carbonate. The Equilibrium Constant A relationship between the concentrations of reactants and products at equilibrium can be determined. An equilibrium will be reached where there is a mixture of N2 H2 and NH3. This equilibrium is only established if the calcium carbonate is heated in a closed system preventing the carbon dioxide from escaping.
As mentioned previously if the system has reached thermodynamic equilibrium this number will be at its minimum possible value. Dynamic Equilibrium can be defined as the state of a given system in which the reversible reaction taking place in it stops changing the ratio of reactants and products but there is a movement of substances between the reactants and the products. When a system consists of competing forward and reverse reaction rates the reaction will proceed until chemical equilibrium is reached. Under such conditions the reactants and products are in chemical equilibrium and will remain so until the system is altered in some way. If a spring is stretched then a force with magnitude proportional to the increase in length from the equilibrium length is pulling each end towards the other. This equilibrium is only established if the calcium carbonate is heated in a closed system preventing the carbon dioxide from escaping. In a chemical reaction chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time so that there is no observable change in the properties of the system.












/NashEquilibrium2-cbc58a27a37a4aab9585c3fc87938509.png)
















Post a Comment for "Solution Equilibrium Is Reached In The System When"